
|
KENT GEOLOGISTS' GROUPAtoms, Elements and Compounds |

|
| Home |
| Organisation |
| About the KGG |
| How to Join |
| Indoor Meetings |
| Field Meetings |
| Fossils |
| Minerals |
| Kent Geology |
Atoms and Elements
An atom consists of two main parts. Firstly a nucleus in which protons, having a positive charge, and neutrons having no charge, are tightly bound together. Secondly, surrounding the nucleus, are one or more electrons in shells, each of which has an associated energy level. The number of electrons is always equal to the number of protons, so the atom has no resultant charge.
Atoms are very very small, typically about one ten millionth of a millimetre in diameter! The nucleus is even smaller; the diameter of the atom being about 10,000 times that of the nucleus. Because atoms are so small their dimensions are measured in nanometres. A nanometre is one millionth of a millimetre. The masses of the proton and neutron are approximately equal and are about 1800 times the mass of an electron.
The simplest element, Hydrogen, has one proton and one electron.
The next element, Helium, has two protons, two neutrons and two electrons.
Electrons are located in orbits or shells, each of which has a maximum complement of electrons. The numbers are two in the first, eight in the second, eight in the third and so on. When the outer shell has a full complement of electrons the element is chemically inactive. These elements are the Noble (or inert) Gases. From stability considerations the outer electron shell of other elements is 'incomplete'. These elements tend to react with other elements to achieve a complete outer shell by giving up, acquiring or sharing outer shell electrons. Chemical reactions between elements always involve the outer shell electrons. These are called the 'valency electrons'. The total number of electrons is called the 'atomic number' of the element.
Compounds
Compounds are products formed by chemical reaction between elements. A compound is not a mixture but a new substance, having different properties from its constituent elements.
For example, sodium chloride, common salt, is a compound of sodium (a soft silvery metal) and chlorine (a greenish gas). It doesn't look or behave like either of its constituents.
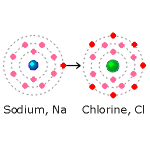 Explained very simply, sodium has atomic number 11. It therefore
has 11 electrons of which 2 are in the innermost shell, 8 in the
second shell and 1 in the third. A complete outer shell would
have 8 electrons. Sodium has one electron surplus to that
required for stability and will react with other atoms. The
sodium atom is shown in the diagram on the left with a blue nucleus.
Explained very simply, sodium has atomic number 11. It therefore
has 11 electrons of which 2 are in the innermost shell, 8 in the
second shell and 1 in the third. A complete outer shell would
have 8 electrons. Sodium has one electron surplus to that
required for stability and will react with other atoms. The
sodium atom is shown in the diagram on the left with a blue nucleus.
Chlorine (shown in the diagram with a green nucleus} has atomic number 17; that is, it has 17 electrons. Two electrons are in the first shell, 8 are in the second shell, leaving 7 electrons in the third shell. This is one short of the 8 electrons needed for a complete shell and therefore for stability.
When sodium and chlorine combine chemically to form sodium chloride, the 'spare' electron in the outer shell of the sodium atom is given up to the outer shell of the chlorine atom. This gives both atoms a complete outer shell with 8 electrons, thus forming a stable compound.
In losing an electron the sodium atom becomes positively charged and forms a sodium ion, written Na1+.The chlorine atom, acquiring an electron, becomes negatively charged and forms a chlorine ion, written Cl1-. In solution, ions combine, negative charge attracting positive charge to form compounds. The way in which atoms and ions combine is determined by chemical bonding (see link below).
The difference between the number of electrons in the outer shell of an atom and that required for a complete outer shell is a measure of an atom's ability to combine with others and is called its 'valency'. Sodium and chlorine (having one spare electron and one electron short respectively) have a valency of one.
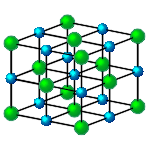
In simple terms, atoms can be thought of as spheres of different sizes. In forming compounds, these spheres pack together in the most compact configuration possible. In sodium chloride, the sodium and chlorine atoms form a cubic, crystal lattice as shown in the figure on the right. The compounds that can be formed will depend on the relative sizes of the atoms. If the atoms trying to form a compound are too far apart the bonding may be too weak; when close enough to form an adequate bond they may interfere. Thus not all atoms are compatible to form compounds even if their valencies suggest that it would be possible.
Rock - Forming Silicates
Silicates are the most abundant rock-forming minerals. The silicate ion is one in which one silicon atom (valency=+4) is attached to four oxygen atoms (valency=-2). The ion, SiO4 is tetrahedral in shape, that is it has four faces. with the four oxygen atoms occupying the four corners of the tetrahedron and the silicon atom at its centre. Each of the oxygen atoms has a spare valency bond.The way in which the four oxygen atoms of each tetrahedron are shared, as the silicate tetrahedra combine, determines the form of the resultant silicate mineral.
In Nesosilicates (Greek, nesos = an island) such as Olivine, no oxygen links are shared and the SiO4 form is retained.
In Sorosilicates (Greek, soros = a group) such as Melilite or Hemimorphite, two tetrahedra share one oxygen atom to form an Si2O7 ion.
In the Cyclosilicates (Greek, cyclo = a ring) such as Tourmaline, the tetrahedra are joined in rings, each sharing two oxygen atoms.
In the Phyllosilicates (Greek, phyllo = a leaf) such as Talc and Mica, the tetrahedra are joined in sheets, sharing three oxygen atoms.
Finally if all four oxygen atoms of each
tetrahedron are shared, the tetrahedra form a
three-dimensional structure as in the
tectosilicates (Greek, tecto = a
framework).
Quartz and the Feldspars are tectosilicates.
| Minerals | What is Matter? | Why Collect Minerals? | Mineral Gallery | Chemical Bonding |